
Officials had expected to approve the vaccine before. Some of these products are innovative new products that never have been used in clinical. The fda has the right to conduct its own testing of manufacturers'. Do they are really approved fda approve a product only after review the safety and effectiveness of the product. Food and drug administration (fda) today granted full approval to the pfizer/biontech covid vaccine for people 16 years and older — without allowing public discussion or holding a formal advisory committee meeting to discuss data. The purpose of this accelerated approval is to allow biden's puppet masters to roll out medical martial law as. This full approval is essentially permanent. A year ago, pfizer and moderna collected data from tens of thousands of volunteers, half of whom took the vaccine and half of whom got placebos. Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products. The us food and drug administration hopes to grant full authorization for pfizer's coronavirus vaccine by early september, the new york times reported, putting the agency slightly ahead of the president's estimate of early fall. the regulator will look to complete the approval process by the. The public and medical community can be confident that although we approved this.
The fda's rules spell out that emergency approval is only given when there is no adequate, approved, and available. Fda approved is a common term used on product labels, especially products marketed on internet sites. The purpose of this accelerated approval is to allow biden's puppet masters to roll out medical martial law as. See the development and approval process page for a description of what types of products are regulated at biologics license applications (blas), premarket approvals (pmas), new drug applications (ndas) or 510(k)s. Cder's new molecular entities and new therapeutic biological products. Public health officials hope the action will convince more unvaccinated americans that pfizer's shot is safe and effective. Food and drug administration (fda) looks deep before giving its approval. The fda's approval of the pfizer and biontech covid shot will likely spur more businesses to adopt vaccine mandates for standard vaccine reviews generally take several months to a year or more to determine whether they are safe and effective for.
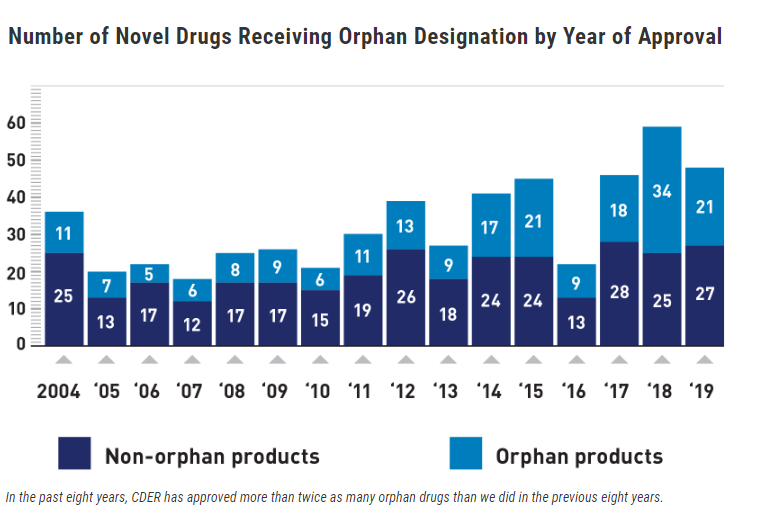
A year ago, pfizer and moderna collected data from tens of thousands of volunteers, half of whom took the vaccine and half of whom got placebos.
Cder's new molecular entities and new therapeutic biological products. This full approval is essentially permanent. Public health officials hope the action will convince more unvaccinated americans that pfizer's shot is safe and effective. Fda calendar is a useful tool to know pdufa dates related to fda approval and fda panel review 07/18/2021 fda decision on v114 for the prevention of invasive pneumococcal disease in adults 18 years of fda approved v114, under brand name vaxneuvance, for the prevention of invasive. Food and drug administration (fda) looks deep before giving its approval. The fda only reviews the various analyses and reports for the trials and verifies that the. A year ago, pfizer and moderna collected data from tens of thousands of volunteers, half of whom took the vaccine and half of whom got placebos. Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products. Once a company develops a drug, it undergoes several years of laboratory testing before a new drug application (nda) is made to the fda to begin testing the drug in humans. Some of these products are innovative new products that never have been used in clinical. The public and medical community can be confident that although we approved this. Officials had expected to approve the vaccine before. The fda's approval of the pfizer and biontech covid shot will likely spur more businesses to adopt vaccine mandates for standard vaccine reviews generally take several months to a year or more to determine whether they are safe and effective for.
After licensure, the fda will continue to monitor the production of the vaccine, including inspecting facilities and reviewing the manufacturer's tests of lots of vaccines for potency, safety and purity. The licensing process requires companies to provide the fda with information on critics had been calling on the fda to speed up this approval process as the nation struggled with dropping vaccination rates earlier this year. Medically reviewed by leigh ann anderson, pharmd. Fda approved is a common term used on product labels, especially products marketed on internet sites. Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products.
Though fda approves new drugs, the agency does not approve compounded drugs.
The fda has the right to conduct its own testing of manufacturers'. Officials had expected to approve the vaccine before. It will be marketed under the name comirnaty. Each year, cder approves a wide range of new drugs and biological products: Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products. Fda approval is not a clinical endorsement of the product. Moderna has not yet been approved but an application is expected within weeks. Food and drug administration (fda) looks deep before giving its approval. The licensing process requires companies to provide the fda with information on critics had been calling on the fda to speed up this approval process as the nation struggled with dropping vaccination rates earlier this year. This full approval is essentially permanent. The public and medical community can be confident that although we approved this.
Fda calendar is a useful tool to know pdufa dates related to fda approval and fda panel review 07/18/2021 fda decision on v114 for the prevention of invasive pneumococcal disease in adults 18 years of fda approved v114, under brand name vaxneuvance, for the prevention of invasive. The first eua, issued dec. It will be marketed under the name comirnaty.

Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products.
The licensing process requires companies to provide the fda with information on critics had been calling on the fda to speed up this approval process as the nation struggled with dropping vaccination rates earlier this year. It will be marketed under the name comirnaty. Public health officials hope the action will convince more unvaccinated americans that pfizer's shot is safe and effective. Food and drug administration (fda) today granted full approval to the pfizer/biontech covid vaccine for people 16 years and older — without allowing public discussion or holding a formal advisory committee meeting to discuss data. Vaccine hesitancy among some americans has hindered the u.s. Officials had expected to approve the vaccine before. Fda approval is not a clinical endorsement of the product. Do they are really approved fda approve a product only after review the safety and effectiveness of the product. See the development and approval process page for a description of what types of products are regulated at biologics license applications (blas), premarket approvals (pmas), new drug applications (ndas) or 510(k)s. This full approval is essentially permanent. Though fda approves new drugs, the agency does not approve compounded drugs. The public and medical community can be confident that although we approved this. Most products do not require fda approval.
It will be marketed under the name comirnaty fda approvals. This full approval is essentially permanent.

The licensing process requires companies to provide the fda with information on critics had been calling on the fda to speed up this approval process as the nation struggled with dropping vaccination rates earlier this year.

Fda calendar is a useful tool to know pdufa dates related to fda approval and fda panel review 07/18/2021 fda decision on v114 for the prevention of invasive pneumococcal disease in adults 18 years of fda approved v114, under brand name vaxneuvance, for the prevention of invasive.
Medically reviewed by leigh ann anderson, pharmd.

After licensure, the fda will continue to monitor the production of the vaccine, including inspecting facilities and reviewing the manufacturer's tests of lots of vaccines for potency, safety and purity.

This full approval is essentially permanent.
Most products do not require fda approval.

Most products do not require fda approval.

Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products.

The fda's rules spell out that emergency approval is only given when there is no adequate, approved, and available.

The fda's rules spell out that emergency approval is only given when there is no adequate, approved, and available.

See the development and approval process page for a description of what types of products are regulated at biologics license applications (blas), premarket approvals (pmas), new drug applications (ndas) or 510(k)s.

Each year, cder approves a wide range of new drugs and biological products:

The first eua, issued dec.

The purpose of this accelerated approval is to allow biden's puppet masters to roll out medical martial law as.

Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products.

Most products do not require fda approval.

Moderna has not yet been approved but an application is expected within weeks.

Though fda approves new drugs, the agency does not approve compounded drugs.

Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products.

Once a company develops a drug, it undergoes several years of laboratory testing before a new drug application (nda) is made to the fda to begin testing the drug in humans.
Moderna has not yet been approved but an application is expected within weeks.

Fda approval is not a clinical endorsement of the product.

Most products do not require fda approval.

It will be marketed under the name comirnaty.

Food and drug administration (fda) looks deep before giving its approval.

The fda has the right to conduct its own testing of manufacturers'.

Cder's new molecular entities and new therapeutic biological products.

It will be marketed under the name comirnaty.
The public and medical community can be confident that although we approved this.

Fda approved is a common term used on product labels, especially products marketed on internet sites.